Diethylstilbestrol syndrome (DES)
What is Diethylstilbestrol Syndrome?
Orphanet — The portal for rare diseases and orphan drugs — defines Diethylstilbestrol Syndrome (DES) as a malformative syndrome reported in the offspring (children and grandchildren) of women exposed to DES (diethylstilbestrol) during pregnancy.
DES is a non-steroidal synthetic estrogen that was widely prescribed to pregnant women between 1940 and 1977 to prevent miscarriage and premature birth.
DES was sold in France under the brand names Distilbène®, Stilbestrol-Borne® and Furostilboestrol® (delayed-release form).
Synonyms:
- DES embryofetopathy
- DES syndrome
- Diethylstilbestrol embryofetopathy
- Distilbene embryofetopathy
ICD-11 (International Classification of Diseases 11th Revision) : LB44.6
Diethylstilbestrol Syndrome is (currently) characterized by:
In men exposed to DES
Reproductive malformations: hypospadias (birth defect in boys in which the opening of the urethra is not located at the tip of the penis), cryptorchidism (undescended testicle) or testicular hypoplasia (poor development of sperm-producing tissue and reduced size of testicles).
We also find epididymal (a narrow, tightly-coiled tube connecting rear of the testicles to the deferent duct) cysts, microphallus (or micropenis, is defined as a stretched penile length of less than 2.5 standard deviations (SDs) below the mean for age), subfertility (reduced fertility).
In women exposed to DES
There is an increased risk of developing clear cell carcinoma of the vagina and cervix (or ACC, a rare variant of adenocarcinoma), cervical or vaginal dysplasia (a precancerous condition in which abnormal cells grow on the surface of your cervix/vagina).
These women may also experience decreased fertility, reproductive malformations which include a vaginal septum, a hypoplastic (underdeveloped) T-shaped uterus or abnormalities of the fallopian tubes, which increase the risk of obstetric complications and miscarriages.
Updating the definition of Diethylstilbestrol Syndrome
Studies carried out at the same time as the definition of Diethylstilbestrol Syndrome have highlighted other disorders which would justify its updating.
Other health problems not listed in Diethylstilbestrol Syndrome are numerous. They include:
Known health effects of DES for both men and women exposed in utero (second generation)
- There is an 11-fold increased risk of any pancreatic disorder and a 7-fold increased risk of pancreatitis1.
- The data from the Hhorages association cohort show a link between prenatal exposure to DES and the appearance of psychological and psychiatric disorders such as: schizophrenia, depression, bipolar disorders, eating disorders, obsessive-compulsive disorder, anxiety, aggressiveness, and suicide attempts2,3,4.
- Epigenetic anomalies: in French psychotic patients exposed to DES in utero, modifications in the expression of two genes were found. These are the ADAM TS9 gene, involved in controlling the shape of the sexual organs during development, and the ZFP57 gene, an alteration of which can impact neurodevelopment.5.
In women exposed to DES in utero
In men exposed to DES in utero
- Increased risk of testicular cancer9.
- A higher prevalence of transgender woman (male to female)10.
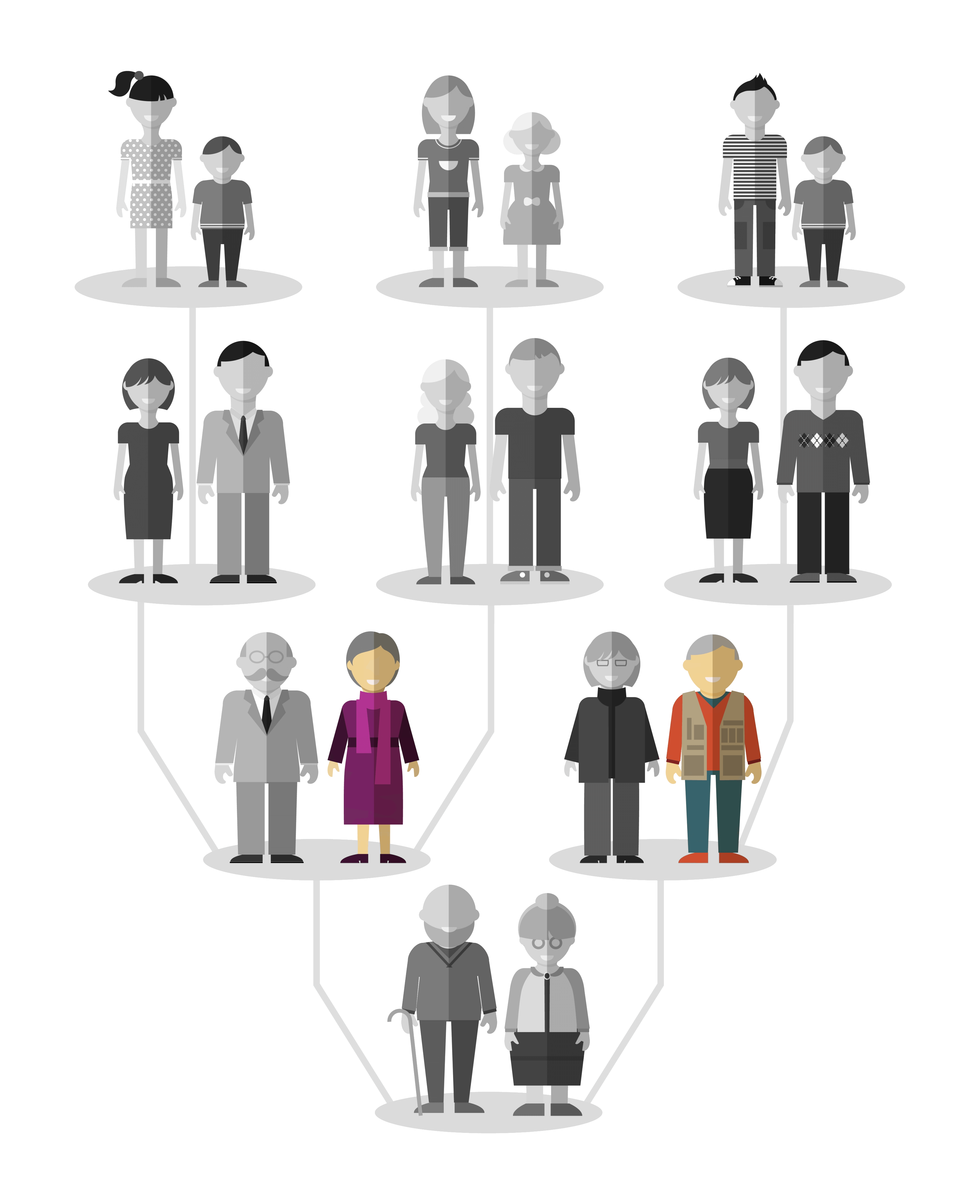
Thus, “DES Daughters” and “DES Sons” are children whose mothers received a drug containing diethylstilbestrol during pregnancy.
They represent the second generation of patients exposed to DES (in color on the tree above), the first generation being represented by the “DES Mothers”.
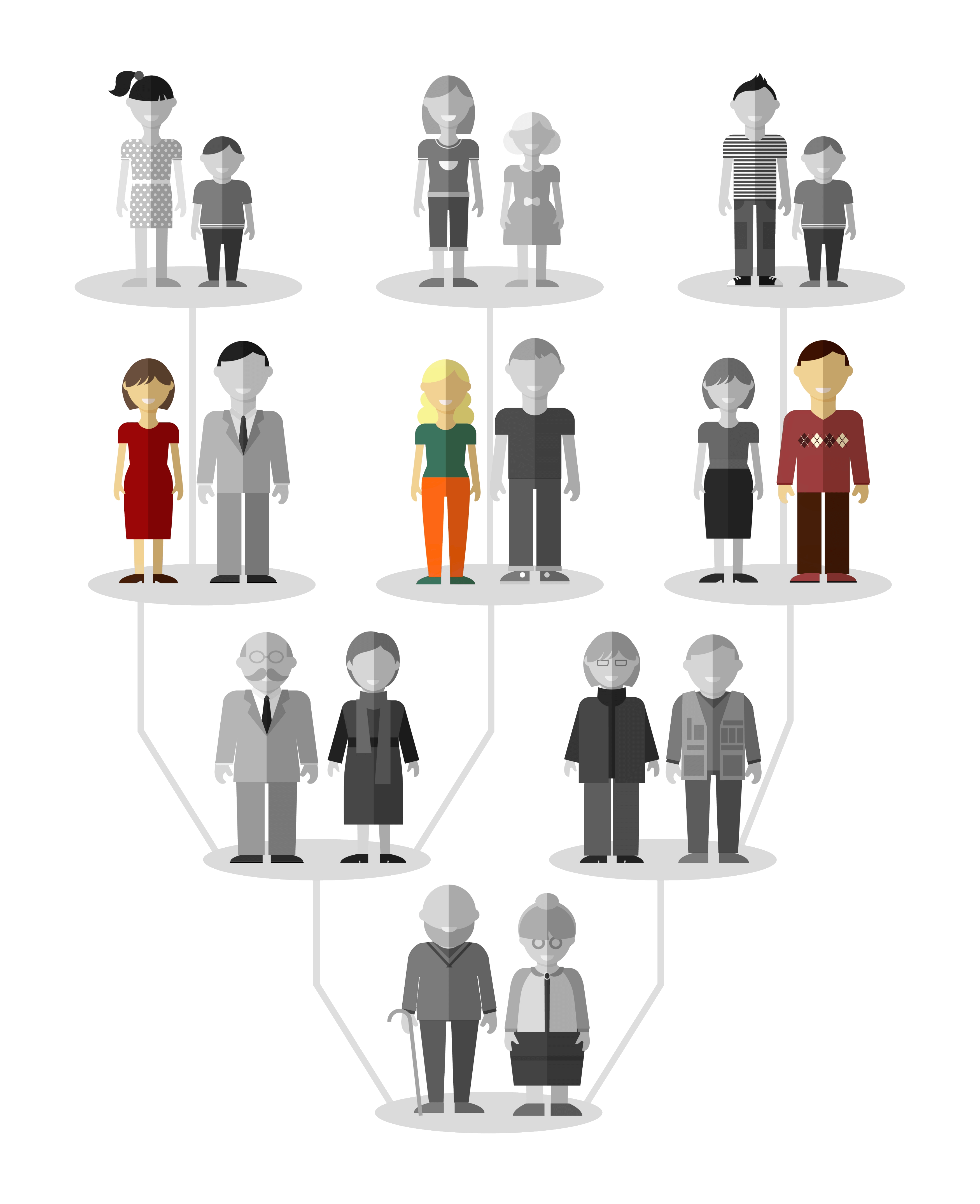
Thus, the “DES Granddaughters” and the “DES Grandsons” are the grandchildren of a woman who received a medication containing diethylstilbestrol during pregnancy.
They represent the third generation of patients affected by DES (in color on the tree above). Their children represent the fourth generation.
In DES Grandchildren, i.e. the third generation
- An increased rate of Cerebral Palsy (CP), related to premature, even very premature births11.
- A significant increase of attention deficit / hyperactivity disorder or ADHD — a neurodevelopmental disorder12.
- An increased risk of cardiovascular malformations13.
- An increased number of esophageal atresia, malformations of the oral cavity, and abnormalities of the musculoskeletal system13,14.
In DES Granddaugthers
- Uterovaginal aplasia has been found, i.e. MRKH syndrome (Mayer-Rokitansky-Küster-Hauser syndrome), that is to say aplasia of the uterus and the upper two thirds of the vagina.15.
- Precocious puberty: breast development in girls less than 8 years, pubic or underarm hair, start of menstruation before age 10...16.
- Endometriosis7.
- Irregular menstrual cycle, amenorrhea17.
- Risk of ectopic pregnancy, premature deliveries17.
- 3 cases of ovarian cancer have been reported by the NCI in: two women aged 20 and 22 years included in the cohort, and one girl too young to be included. These are really rare cancers in young women18.
Prevalence of Diethylstilbestrol Syndrome
In France, the number of women given diethylstilbestrol (Distilbène®, Stilbestrol-Borne® or Furostilboestrol®) is estimated at 200,000 and the number of children exposed in utero at 160,000.
As there is no patient register, the prevalence of the disease is unknown.
This is why we have launched a national census of patients affected by diethylstilbestrol, below which you will find the resulting statistics.

Diagnosis and management of Diethylstilbestrol Syndrome
It is currently impossible for a patient to be diagnosed.
Indeed, there is no Reference Center for this rare iatrogenic disease, and no French National Diagnostic and Care Protocol (PNDS).
The PNDS is a reference document for health professionals, written by the experts of the Reference Center using a methodology proposed by the High Authority of Health (HAS).
The objective of a PNDS is to explain to the professionals concerned the current optimal diagnostic and therapeutic care and the care path of a patient suffering from a rare disease given.

The D.E.S is it association has set itself the objective of filling all these gaps, starting by joining one or more Reference Centers, in order to constitute a group of experts and work, hand in hand, on the development of a PNDS for Diethylstilbestrol Syndrome (DES).
Notes & references
- ↑ a ↑ b Troisi, R., Hyer, M., Titus, L., Palmer, J., Hatch, E., Huo, D., Hoover, R. (2020). Prenatal diethylstilbestrol exposure and risk of diabetes, gallbladder disease, and pancreatic disorders and malignancies. Journal of Developmental Origins of Health and Disease, 1-8. doi:10.1017/S2040174420000872
- ↑ O'Reilly EJ, Mirzaei F, Forman MR, Ascherio A. Diethylstilbestrol exposure in utero and depression in women. Am J Epidemiol. 2010 Apr 15;171(8):876-82. doi: 10.1093/aje/kwq023. Epub 2010 Mar 23. PMID: 20332145; PMCID: PMC2877444.
- ↑ "Distilbène et Troubles du Comportement : Hasard ou Evidence ?", présentation du Dr Marie-Odile Soyer-Gobillard, directeur de recherche émérite honoraire au CNRS et présidente de Hhorages-France (Halte aux HORmones Artificielles pour les GrossessES) lors du colloque du 18 juin 2011 à l'Assemblée Nationale "Distilbène/Médiator, 1977 - 2009, 2 époques, 2 scandales... Et demain ?".
- ↑ "Exposition in utero au diéthylstilbestrol (DES) troubles psychiques confirmés" Revue Prescrire 2018 ; 38 (412).
- ↑ Rivollier F, Chaumette B, Bendjemaa N, Chayet M, Millet B, Jaafari N, Barhdadi A, Lemieux Perreault LP, Provost S, Dubé MP, Gaillard R, Krebs MO, Kebir O. Methylomic changes in individuals with psychosis, prenatally exposed to endocrine disrupting compounds: Lessons from diethylstilbestrol. PLoS One. 2017 Apr 13;12(4):e0174783. doi: 10.1371/journal.pone.0174783. ECollection 2017.
- ↑ Stillman RJ, Miller LC. Diethylstilbestrol exposure in utero and endometriosis in infertile females. Fertil Steril 1984 ;41:369–72.
- ↑ a ↑ b Gaspari, L., Soyer-Gobillard, MO., Paris, F. et al. Multigenerational endometriosis : consequence of fetal exposure to diethylstilbestrol ?. Environ Health 20, 96 (2021). https://doi.org/10.1186/s12940-021-00780-5
- ↑ Find a complete list of studies on Journal of a DES Daughter
- ↑ Hom M, Sriprasert I, Ihenacho U, Castelao JE, Siegmund K, Bernstein L, Cortessis VK. Systematic Review and Meta-analysis of Testicular Germ Cell Tumors Following In Utero Exposure to Diethylstilbestrol. 2019.
- ↑ Gaspari, L.; Soyer-Gobillard, M.-O.; Kerlin, S.; Paris, F.; Sultan, C. Early Female Transgender Identity after Prenatal Exposure to Diethylstilbestrol: Report from a French National Diethylstilbestrol (DES) Cohort. J. Xenobiot. 2024, 14, 166-175. https://doi.org/10.3390/jox14010010
- ↑ Hatch EE, Troisi R, Wise LA, Titus-Ernstoff L, Hyer M, Palmer JR, Strohsnitter WC, Robboy SJ, Anderson D, Kaufman R, Adam E, Hoover RN. Preterm birth, fetal growth, and age at menarche among women exposed prenatally to diethylstilbestrol (DES). 2011.
- ↑ Kioumourtzoglou MA, Weisskopf MG. Grandmaternal Diethylstilbestrol and Attention-Deficit/Hyperactivity Disorder in Children-Reply. JAMA Pediatr. 2018 Dec 1;172(12):1204-1205. doi: 10.1001/jamapediatrics.2018.3743. PMID: 30383104.
- ↑ a ↑ b Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, Hoover RN. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). 2010.
- ↑ Michel Tournaire , Sylvie Epelboin, Emmanuel Devouche, Géraldine Viot, Jérôme Le Bidoi, Anne Cabau, Annabel Dunbavand, Anne Levadou. Adverse health effects in children of women exposed in utero to diethylstilbestrol (DES). 2016.
- ↑ Wautier A, Tournaire M, Devouche E, Epelboin S, Pouly JL, Levadou A. Genital tract and reproductive characteristics in daughters of women and men prenatally exposed to diethylstilbestrol (DES). Therapie. 2020 Sep-Oct;75(5):439-448. doi: 10.1016/j.therap.2019.10.004. Epub 2019 Nov 1. PMID: 31806244.
- ↑ Assemblée Nationale - Question N° : 109961 http://questions.assemblee-nationale.fr/q13/13-109961QE.htm
- ↑ a ↑ b Titus L, Hatch EE, Drake KM, Parker SE, Hyer M, Palmer JR, Strohsnitter WC, Adam E, Herbst AL, Huo D, Hoover RN, Troisi R. Reproductive and hormone-related outcomes in women whose mothers were exposed in utero to diethylstilbestrol (DES): A report from the US National Cancer Institute DES Third Generation Study. Reprod Toxicol. 2019 Mar;84:32-38. doi: 10.1016/j.reprotox.2018.12.008. Epub 2018 Dec 27. PMID: 30594671; PMCID: PMC6382553.
- ↑ Sylvie Epelboin, Michel Tournaire, Emmanuel Devouche. Exposition au Distilbène® in utero : effets transgénérationnels. Médecine de la Reproduction. 2022;24(1):37-48. doi:10.1684/mte.2022.0875
- The images on this page are the creation of rawpixel.com / Freepik
Sign in to your account
WARNING
As our website is regularly targeted by cyberattacks, some pages — and we're sorry about this — may not be displayed correctly.
If so, we recommend that you try again later.
Thank you for your understanding and patience.
Bylaws
Article 1 – Name of Organization
The name of the organization is D.E.S is it.
D.E.S is it is a Nonprofit Organization based on french law No. 40-484 of July 1, 1901.
Article 2 – Specific Purposes
The specific objectives and purposes of our Organization are:
- Defend the interests of DES victims (diethylstilbestrol), all generations and sexes combined;
- Supply information victims of DES, their entourage and health professionals on the consequences and transgenerational effects of DES;
- Inform victims of the DES of possible remedies, including legal ones, to obtain compensation for the drug-related injuries: bodily, moral and financial;
- Raise awareness amongst the general public to help prevent healthcare drug scandals;
- Create a Solidarity networks, mutual aid, support for DES victims;
- Connecting with other Organizations engaged in defending:
- interests of DES victims;
- interests of victims Big Pharma;
- interests of victims of endocrine disruptors;
- and more broadly, interests of victims of medical errors
- Collect data in order for studies, research and evaluation work;
- Publish press releases and guides;
- Cooperate with international scientific research: participation in scientific studies on the transgenerational effects of DES;
- Contact the competent authorities to submit them proposals about:
- Injury recognition;
- The creation of DES status;
- The creation of a DES diagnosis;
- DES healthcare;
- Compensation for victims of DES.
Article 3 – Headquarters
Organization headquarters: LYON, FRANCE.It can be moved by decision of the President.
Article 4 – Duration
The duration of the organization is indefinite.
Article 5 – Classes of Members
- Founding members: Natural persons interested in the purposes of the Organization, adhering to the Bylaws and its internal regulations, and whose names appears in the appendix to these Bylaws. Founding members may co-opt other founding members during the life of the Organization. The founding members participate in the General Meeting with deliberative voice.
- Regular members: Regular members are natural or legal persons interested in the purposes of the Organization in accordance with these Bylaws and its internal regulations, and who submit to a membership application and the payment of such dues and fees as apply at the time. They are members of the General Meeting with deliberative voice.
- Honorary members: Honorary members are individuals who have been recognized by the Organization for outstanding contribution to the Organization. They are nominated by the President and the Vice-President. They have no deliberative voice.
- Benefactor Members: Any natural or legal person choosing to contribute additional financial support to the Organization, by making a donation worth 150€ or more, in addition to their regular membership become a Benefactor Member for the current calendar year.
Article 6 – Membership
Membership is open to all victims of DES and their close relatives. Other Membership applications will be considered on a case-by-case basis.
To be part of the Organization, it is necessary to be approved by the Vice-President who rules on Membership applications presented.
Article 7 – Dues
Each member must pay, within the time and on the conditions set forth in these Bylaws, the dues. There may be different dues for different classes of Members. The amount is set by the Board.
Article 8 – Termination or Suspension of Membership
Causes of Termination or Suspension:
- Resignation of the member; Any member may voluntarily withdraw from membership at any time. The letter may be addressed to the President;
- Death of the member;
- Any member who has not paid their dues;
- Expiration of the fixed term of the membership, unless the membership is renewed before its expiration;
- Termination of the legal existence of a member that is not a natural person;
- Expulsion of the member. If a member would affect an important, substantial interest of the Organization, he can be expelled or suspended.
The member to be expelled or suspended is given notice, including a statement of the reasons for the expulsion or suspension.
He is given an opportunity to be heard by the person or body deciding the matter, orally or in writing.
Founding member are permanent members with the voting privileges Any founding member can resign from her/his position. A founding member may be removed by unanimous vote of other founding members for engaging in activities contrary to the Bylaws of the Organization.
Article 9 – Affiliation
The Organization may affiliate with or otherwise participate in the activities of other Organizations, or other Groups, by decision of the Office.
Article 10 – Resources
The resources of the Organization include:
- Dues amount;
- Founding Members contributions;
- All resources, including donations, legacies and life annuities, and all services, the obtaining of which is not contrary either to the law, to the regulations in force, or to the purposes of this Organization;
- Financial aid that the Organization can receive from patrons because of its purpose under the conditions provided for in article 238 bis of the Code Général des Impôts;
Article 11 – General Meetings
The General Meeting of the Organization shall be held in May of each year at a place and on a date to be selected by the Office.
All members in good standing may attend of the General Meeting.
The Organization shall give Members written notice of the date, time and place of each General and Special Members’ Meeting. Such notice shall be given, either personally, by mail or electronic means, not less than fifteen (15) days before the date of the meeting.
A quorum for the General Meeting of the Organization shall be no less than 30% members in good standing who shall be present in person or represented by proxy at such duly called meeting. A member cannot hold more than 2 mandates.
If quorum is not met, the Meeting is reconvened within a period of fifteen to thirty days and, in this second meeting, it validly deliberates by an absolute majority, whatever the number of members present or represented.
The President, assisted by the Vice-President, preside the General Meeting.
The Treasurer shall submit the annual financial report.
Voting in all General Meetings shall be by voice vote, roll call vote, show of hands, electronic vote or mail. All matters will be decided by a majority vote of both those in attendance and those who have submitted proxy votes in advance.
The President may cast the decisive vote in the event of a tie.
Any decisions taken by General Meeting shall be binding on all members, including any absent or dissenting members and any that were incapable of voting.
Article 12 – Special Meetings
Special Meetings of the members may be called by the President or not less than one-third (1/3) of the members having voting rights, n the same way as General Meeting, only for amendment of the Bylaws or the dissolution or for the merger of the Organization.
The call for the Special Meeting shall be issued by the President, unless at least one-third (1/3) of its member requesting the meeting.
A quorum for the Special Meeting of the Organization shall be no less than 50% members in good standing who shall be present in person or represented by proxy at such duly called meeting.
All matters will be decided by a two-thirds majority vote of both those in attendance and those who have submitted proxy votes in advance.
The Minutes of the meeting will be recorded.
Article 13 – Officers
General Meeting will nominate and elect from within their ranks, the members of the Office enumerated below:
- A President;
- A Vice-president / A Treasurer;
- A Secretary, if necessary;
- A Communication representative, if necessary.
The members of the Office, each serving three-year terms, are re-eligible;
To stand for election, you must have been a member of the association for at least one year, have attended at least one General Meeting and be up-to-date with their membership fees. A member of the Executive Committee or Board of Directors of an association with a similar purpose may not stand for election.
The Office shall have the widest powers and authority to manage, direct, administer and represent the Organization within the limits of the Purpose of the Organization and within the framework of the resolutions adopted by the General Meeting.
The Office are convened as needed by the President or at the request of a quarter of the members of Boards of Directors.
Article 14 – President
The President, and in the event of incapacity the Vice-President, shall represents the Organization and be held accountable to the Law.
The President shall, when present, preside at all meetings of the Organization, and have power to call Special meetings of the Organization or Office, as provided herein, for any purpose or purposes.
The President shall serve as a member of the Office in accordance with the Organization Bylaws and shall report actions to the Office and membership.
He shall perform duties ordinarily incident to his office, shall recommend such action as he deems proper, and sign such papers of the Organization as he may be authorized or directed by the Office.
Article 15 – Vice-President
The Vice-President designated by the Office shall assist the President in all duties.
In the absence or disability of the President, the Vice President shall perform all duties of the President. When so acting, a Vice President shall have all powers of and be subject to all restrictions on the President. The Vice President shall have such other powers and perform such other duties as the Office or the Bylaws may prescribe.
Article 16 – Treasurer
The Treasurer shall oversee and ensure the safe custody of all funds, securities, evidence or indebtedness, and other valuable documents of the Organization and whenever required by the Office or the President, render a statement of cash accounts;
The Treasurer keep or cause to be kept such books and records as will show a record of expenses, losses, gains, assets and liabilities of the Organization; present and make to the annual meeting of the Organization and all Office meetings a report of the finances of the Organization; and to perform all other duties incidental of the office of Treasurer.
Article 17 – Secretary
The Secretary shall give, or cause to be given, notice of all meetings of the Organization and all other notices required by law or by these Bylaws. He shall archive all important documents for the Organization life.
In the event of the Secretary’s absence or inability to do so, such notice may be given by any person directed by the President, the Office, or members upon whose requisition the meeting is called as provided by these By-Laws, and record all of the proceedings of the meetings of the Association or Office, and perform such other duties as may be assigned to him or her by the Office or the President.
Article 18 – Communication representative
Communication representative is responsible for planning and implementing communications inside and outside the Organization.
Article 19 – Indemnifications
No indemnifications: The Organization is too small to have the financial means to pay legal bills of Board members or purchase Directors and Officer insurance.
Article 20 – Standing rules
Standing rules may be established by the Office. General Meeting must show members approved the new Standing rules.
Standing rules are rules or resolutions to further supplement and clarify the Bylaws. Such rules shall be in addition to the Bylaws and shall not be construed to change or replace any Bylaws.
Article 21 – Dissolution
The Organization may be dissolved by resolution of the Special meeting adopted at any time, in accordance with the law and for the reasons provided for therein.
The Special meeting resolution shall then appoint a liquidator.
Upon dissolution, the net assets of the Organization, if any, shall not inure to the benefit of any private individual, unincorporated organization or corporation, including any member, officer or director of the Organization, but shall be distributed to a group or other organization pursuing an identical goal or to charitable works, in accordance with article 9 of the law of July 1, 1901.
Article 22 – Donations
The Organization shall provide its Financial and Accounting Records on any requisition from the Administrative authorities for inspection regarding the use of donations that it would be authorized to receive, and to report to them on its operation.
Appendices to Bylaws
Appendix I – List of the Founding Members
- Déborah Maitrejean
- Salomé Maitrejean
Appendix 2 – List of the Officers
- Déborah Maitrejean: President
- Salomé Maitrejean: Vice-President and Treasurer
Rules and Regulations
Rules and Regulations
Preamble
These Rules and Regulations are established in accordance with article 20 of the Bylaws of the Association. Its purpose is to specify the bylaws of the Association D.E.S is it, who has its seat in Lyon, France, and whose purposes are set forth in Article 2 of the Bylaws.
Article 1 – Membership Approval
a) A minor can join the association but he must provide a written consent from his parents.
b) Submission of erroneous, incomplete or illegible information on the membership application may result in rejection.
c) The member guarantees, on his membership application, the accuracy, the honesty and the truthfulness of the information.
Article 2 – Obligations of members
a) Membership in the Association implies compliance with the Bylaws and the Rules and Regulations.
b) Membership presupposes the absence of conflict of interest.
c) The member ensures that his e-mail address is always up to date so that the association can communicate important information to him.
d) The member undertakes to indicate his presence or absence for each meeting.
Article 3 – Resignation – Suspension and expulsion – Death of a member
a) A member may resign at any time by delivering a written resignation to the President of the Board. Such resignation shall be effective upon receipt by the Association. It does not have to be motivated.
b) In the event of the death of the member, no right in membership accrues to his heirs or anyone else. Membership of the Association is personal and non-inheritable.
Their application will have to be accepted under the same conditions as those of new members.
c) Pursuant to Article 8 of the Bylaws, the board of directors may expel or suspend any member for serious offenses. Reasons for expulsion or suspension may include, but not be limited to:
-
having made material misstatements on the application for membership;
-
non-participation in the activities of the Association;
-
having been found guilty by a court of competent jurisdiction of a crime punishable by imprisonment for more than one year, a felony, or any crime involving moral turpitude;
-
any action likely to harm, directly or indirectly, the activities of the Association or its reputation, as well as its members;
-
having failed to abide by the Bylaws or these Rules and Regulations;
d) No member may transfer for value or otherwise a membership or any right arising therefrom, and all rights of membership shall cease upon the member’s death, resignation, expulsion, termination, or dissolution.
Dues, donations and service fees paid shall not be refundable.
Article 4 – Access to the private area of the association’s website
a) After his membership has been approved, the member can access a private area, which he must activate, where he can, among other things: find documents related to his membership, access and participate in the Association's forums, consult information, answer polls, receive notifications.
The first time he logs in, he is invited to introduce himself on the forums.
b) The member can delete his private account from the website at any time. He remains a member of the Association for the current calendar year.
c) Any member whose expulsion has been decided will be immediately blocked access to the private area of the website until he provides explanations. In the event that the expulsion of the member is decided, his access to the private area of the site is definitively removed.
d) As certain technical matters may be beyond our control, we cannot guarantee that you will have uninterrupted access to the website at all times.
If the member has specific questions or comments about the design or functionality of the website, he can contact the webmaster directly at the following address: [email protected]
Article 5 – Amendments
These Rules and Regulations may be only amended, repealed, or altered in whole or in part by the members of the Board.
Appendix
In order to find out more about the way we collect and process your personal data, the rights you have and how to exercise these rights with ease, the member is encouraged to read D.E.S is it’s Privacy Policy, accessible at the following address: https://www.des-is-it.org/en/privacy-policy
Legal Notices
Legal Notices
Last updated: July 17, 2024.
D.E.S is it is a Nonprofit Organization based on french law No. 40-484 of July 1, 1901. RNA: W691101273.
The website https://www.des-is-it.org is owned by D.E.S is it, headquartered in Lyon. All mail should be sent to the following address:
- D.E.S IS IT BP 7018 69007 699280, France.
To learn more about our Privacy Policy, please click the link below:
To learn more about our Terms of use, please click the link below:
Hosting Provider:
- PlanetHoster 4416 Louis-B.-Mayer – Laval, Québec – Canada.
You are welcome to contact us at hello[at] des-is-it.org at any time with any questions.
The images of vector types are for the most part the creation of rawpixel.com / Freepik
Any reproduction of this website, even partial, is strictly prohibited without the prior authorization of D.E.S is it.
Terms of Use
Terms of Use
ARTICLE 1: Object
These Terms of Use apply to the site: https://www.des-is-it.org
Last updated: January 08, 2022
The purpose of these Terms of Use is to provide the legal framework for the provision of services on the https://www.des-is-it.org website and their use by the "User".
The Terms of Use must be accepted by any User wishing to access the site. They constitute the contract between the site and the User. Access to the site by the User means acceptance of these Terms of Use.
Eventually :
In case of non-acceptance of the Terms of Use stipulated in this contract, the User must waive access to the services offered by the site.
D.E.S is it reserves the right to modify unilaterally and at any time the contents of these Terms of Use.
These Terms of Use complete the Privacy Policy which you can consult on the following page: Privacy Policy.
ARTICLE 2: Definitions
The purpose of this clause is to define the various essential terms of the contract:
- User: this term refers to any person who uses the site or any of the services offered by the site.
- User content: these are the data transmitted by the User within the site.
- Member: the User becomes a member when identified on the site.
- Login and password: this is all the information needed to identify a user on the site. The username and password allow the User to access services reserved for members of the site. The password is confidential.
ARTICLE 3 : Accès aux services
Utilisateur
L’Utilisateur du site https://www.des-is-it.org a accès aux services suivants :
- Contacter L’Éditeur ;
- Déposer une candidature pour devenir bénévole ;
- Adhérer à l’association ;
- Demander le renvoi de l'e-mail permettant de créer son compte sur le site (après adhésion) ;
- Faire un don à l’association. Le formulaire est également accessible depuis la plateforme HelloAsso : https://www.helloasso.com/associations/d-e-s-is-it/formulaires/1/widget ;
- Signaler un problème technique ;
- Formulaire d’oubli de son mot de passe.
Tout Utilisateur ayant accès a internet peut accéder gratuitement et depuis n’importe où au site. Les frais supportés par l’Utilisateur pour y accéder (connexion internet, matériel informatique, etc.) ne sont pas à la charge de l’Éditeur.
Adhérent
L’adhésion est strictement réservée aux personnes faisant usage de la langue française. Pour devenir Adhérent du site https://www.des-is-it.org :
- L’Utilisateur doit compléter et soumettre le formulaire d’adhésion, accessible sur la page d’adhésion du site : https://www.des-is-it.org/fr/adhesion ;
- L’Éditeur envoie alors, de manière automatisée, un e-mail à l’Utilisateur contenant un lien unique sécurisé ;
- L’Utilisateur doit cliquer sur le lien de l’e-mail ; le lien le redirige alors sur la page d’inscription du site, où il se choisit un mot de passe et un pseudonyme. Il est invité à ne pas entrer sa véritable identité ;
- Une fois le formulaire d’inscription soumis, il peut accéder à son espace personnel en se connectant.
L’Adhérent a accès aux services suivants :
- Publication de commentaires sur le blog ;
- Dépôt de « likes » sur les articles du blog ;
- Forums de discussion : publications de sujets et de réponses aux sujets ;
- Participation aux sondages ;
- Documents relatifs à la vie de l'association ;
- Documents relatifs au D.E.S. ;
- Documents divers ;
- Modifications de ses paramètres.
Tout Adhérent ayant accès a internet peut accéder après connexion et depuis n’importe où au site. Les frais supportés par l’ Adhérent pour y accéder (connexion internet, matériel informatique, etc.) ne sont pas à la charge de l’Éditeur.
L’Adhérent peut modifier, depuis son espace personnel, les données suivantes :
- Adresse e-mail ; tout changement d’adresse e-mail est répercuté automatiquement sur les fichiers adhérents de L’Éditeur, afin de maintenir à jour les coordonnées ; il peut choisir d’afficher ou non son adresse e-mail aux autres adhérents ;
- Mot de passe ;
- Pseudonyme ;
- Avatar : L’Adhérent est invité à choisir un avatar plutôt qu’une photo de lui, afin de garantir, s’il le souhaite, son anonymat ;
- Suppression de son compte adhérent sur le site : ses commentaires postés sur le blog, ses sujets et ses réponses aux sujets des forums de discussion, sont alors attribués à un compte dit « compte supprimé », anonymisant alors ses données, ceci afin de ne pas devoir procéder à la suppression des fils entiers de discussions et maintenir une cohérence de lecture.
Le site et ses différents services peuvent être interrompus ou suspendus par l’Éditeur, notamment à l’occasion d’une maintenance, sans obligation de préavis ou de justification.
ARTICLE 4 : Responsabilité de l’Utilisateur
L’Utilisateur assume l’entière responsabilité de l’utilisation qu’il fait des informations et contenus présents sur le site https://www.des-is-it.org.
Tout usage du service par l’Utilisateur ayant directement ou indirectement pour conséquence des dommages doit faire l’objet d’une indemnisation au profit de L’Éditeur.
ARTICLE 5 : Charte de l’Adhérent
L’Adhérent accepte et s’engage :
- à être à jour de sa cotisation pour pouvoir participer aux services proposés sur le site par L’Éditeur (tels que les forums de discussion, les sondages…) ;
- à ne pas utiliser les coordonnées des adhérents à des fins commerciales ou de démarchage ;
- à signaler tout changement de coordonnées pour la mise à jour de la base de données ;
- à participer, avec bienveillance et dans la bonne humeur, aux services proposés, conscient du travail bénévole accompli ;
- ayant un accès sécurisé au site internet avec des informations réservées aux adhérents grâce à un login et un mot de passe personnel, à ne le diffuser à personne ;
- à ne pas utiliser le contenu des messages des forums afin de protéger la vie privée des adhérents ;
- à ne pas divulguer le contenu des sondages afin de permettre à l’association de mener ses recherches à bien.
Le respect de cette charte est essentiel au bon fonctionnement de notre association.
ARTICLE 6 : Charte d’utilisation du blog
L’Adhérent peut déposer un commentaire en-dessous de chacun des articles du Blog, ainsi que « liker » l’article, sauf dans le cas des articles dans lesquels les commentaires ont été désactivés.
L’Adhérent est responsable du contenu qu’il diffuse sur Internet notamment à travers ses commentaires, et l’Éditeur ne se déclare pas responsable du contenu des commentaires.
L’ Adhérent a été invité à ne pas entrer son véritable nom, et à choisir un pseudonyme lors de son inscription au site. Si l’Adhérent a entré son véritable nom et souhaite supprimer son commentaire, il lui suffit de contacter l’Éditeur pour qu’il supprime le commentaire.
Les commentaires de l’Adhérent sont visibles par tout Utilisateur du site.
L’Éditeur se réserve le droit de modérer les éventuels commentaires qui ne respectent pas les présentes Conditions Générales d’Utilisation, la Nétiquette ou encore les lois en vigueur.
Dans le cas où un Adhérent souhaite signaler un commentaire, il lui suffit de cliquer sur le triangle de signalement prévu à cet effet, afin que l’Éditeur supprime ou modère le commentaire signalé comme indésirable.
ARTICLE 7 : Forums Rules and Terms
Overview and Purpose
D.E.S is it forums are dedicated to developing and maintaining a friendly online community for the victims of D.E.S, where members of all ages feel relaxed and comfortable.
These forums are not a substitute for medical advice, diagnosis or treatment, nor is the information provided herein intended to replace consultation with a qualified physician or other health care provider.
The forum activity allows members of D.E.S is it to exchange ideas by posting comments as part of a 'thread', with the team of D.E.S is it or with other members.
Like any community, D.E.S is it forums have certain standards. When members join our forums, they agree to abide by these rules.
Members must be considerate to others. Repeated violations of these standards may result in a member being barred from entry or participation in the community forums.
Forums Accesses
Only the members of D.E.S is it can join the forums and use these tools to communicate.
Responsibility of Contributors
You are responsible for the content of the messages you post and any consequences that flow from such posting(s);
You are prohibited from posting or transmitting any of the following content:
- Content that violates state or federal law;
- Content that discusses illegal activities with the intent to commit them;
- Content that is libelous or that defames or threatens others;
- Harassing statements;
- Hate speech;
- Content that is obscene;
- Unauthorized copyrighted material or material that infringes another’s intellectual property;
- Content that is confidential or subject to a non-disclosure agreement;
- Advertising or any form of commercial solicitation; If you have a special offer you would like to make directly to forum members, email the Moderator first to discuss it;
- Communications that could be confused with official communications of D.E.S is it;
- Content that is otherwise offensive or objectionable.
Violation of these rules may result in disciplinary measures, which may ultimately include being banned from the Forums of D.E.S is it.
Usage Tips
Please respect the privacy of others. Do not give out or re-post ANYONE'S personal information such as names, phone numbers, addresses, email addresses, etc. This applies to your own information and that of other users.
All the forums are categorized by categories. Please post your questions or posts in the appropriate forum. Answers to many of the questions you may have can be found in the Faq Section or from other posts on the forums. If you are still having questions, problems... feel free to post yours.
When you formulate medical and/or scientific theories on the discussion forums, these must have been checked beforehand.
If it is possible, each contributor of the forums shall cite the sources (links, books, research resources, medical journals, etc.) of the medical and/or scientific data posted on the forums, if which do not coming from his/her own personal experiences.
Disclaimer
D.E.S is it makes no guarantees as to the accurateness, quality, or completeness of the information, and D.E.S is it shall not be responsible or liable for any errors, omissions, or inaccuracies in the information available on these forums.
We do not control, are not responsible for and make no representations or warranties with respect to any user or user conduct.
You are solely responsible for your interaction with or reliance on any user or user conduct. You are also responsible for your own conduct and activities on, through our forums.
Readers are also noticed that each contributor speaks for himself even if he is a medical professional or legal professional.
Medical Disclaimer
We are not doctors and we do not provide medical advice. The content on our forums are is intended to be used for educational, informative, and entertainment purposes only. Any information on our forums are not intended to be, should not be interpreted as, or used as a substitute for, medical advice or a diagnosis of any health or fitness problem, condition or disease; or a recommendation for a specific test, doctor, care provider, procedure, treatment plan, product, or course of action.
You should obtain any additional information necessary to make an informed decision prior to utilizing any specific treatments.
Moderation
We have the right, but not the obligation, to regulate content (which includes but is not limited to postings, text, images, video, ads, messages and any other user communications ("content")) posted to, stored on or transmitted via our forums by any user (or any other third party in any manner); to regulate conduct (including but not limited to any authorized or unauthorized access to or use of our forums) by any user (or any other third party in any manner); and to enforce these Rules and Terms, for any reason and in any manner or by any means that we, in our sole discretion, deem necessary or appropriate.
You can report a topic by clicking the button on the top right of the topic, and an answer by clicking the button on the bottom right of the answer.
If your thread has been published in the wrong forum, the moderator can moved it to another forum or merged with an existing thread in the new forum.
Messages typed in in abbreviated language (For example; "UR" instead of "you are") or completely in capital letters could be edited by the moderator.
Repetitive posting of the same topic or text or nonsensical posts those have no substance could be deleted by the moderator.
The moderator of these forums have the right to modify, delete, edit or close any topic.
If you have any questions concerning this, please do not start a new thread, but rather send an email to the moderator at: [email protected]
Confidentiality of the messages
Due to the private nature of the forums, none of the contributions can be found on a search engine, or be visible to an external audience.
Editing and Deleting your Posts
Members are free to leave and updates theirs posts, but not free to delete the history of a discussion. Members wishing to leave the forums of D.E.S is it can do so by deleting their account at any time.
Their account will then be set to “inactive” and their posts will remain.
ARTICLE 8 : Politique concernant les sondages
Il s’agit de courtes enquêtes, limitée à quelques questions, disponibles directement sur le site https://www.des-is-it.org.
L’Adhérent s’engage à répondre de manière consciencieuse et honnête aux questions des sondages ;
Pour en savoir plus sur la confidentialité de votre participation aux sondages, consultez notre Politique de protection des données personnelles.
ARTICLE 9 : Politique concernant les pourriels
Il est strictement interdit d’utiliser le site ou tout service proposé sur le site à des fins d’activités illégales reliées aux pourriels, incluant la collecte d’adresses et d’informations personnelles d’autrui ou l’envoi de courriels commerciaux de masse
ARTICLE 10 : Propriété intellectuelle
Les contenus du site https://www.des-is-it.org (logos, textes, éléments graphiques, vidéos, codes sources etc.) son protégés par le droit d’auteur, en vertu du Code de la propriété intellectuelle.
L’Utilisateur devra obtenir l’autorisation de l’Éditeur du site avant toute reproduction, copie ou publication de ces différents contenus.
Ces derniers peuvent être utilisés par les utilisateurs à des fins privées ; tout usage commercial est interdit.
ARTICLE 11 : Limitations contractuelles
Les domaines vers lesquels mènent les liens hypertextes présents sur le site n’engagent pas la responsabilité de l’Éditeur de https://www.des-is-it.org, qui n’a pas de contrôle sur ces liens.
Il est possible pour un tiers de créer un lien vers une page du site https://www.des-is-it.org sans autorisation expresse de L’Éditeur.
ARTICLE 12 : Évolution des Conditions générales d’utilisation
L’Éditeur se réserve le droit de modifier les clauses de ces Conditions générales d’utilisation à tout moment et sans justification.
ARTICLE 13 : Indépendance des clauses
Si une des présentes clauses est reconnue comme illégale, déclarée nulle ou inapplicable, en tout ou en partie, la portion inapplicable n’affectera pas la validité et l’applicabilité des autres clauses des présentes Conditions générales d’utilisation.
ARTICLE 14 : Durée du contrat
La durée du présent contrat est indéterminée. Le contrat produit ses effets à l’égard de l’Utilisateur et de l’Adhérent à compter du début de l’utilisation du service.
ARTICLE 15 : Droit applicable et juridiction compétente
Le présent contrat dépend de la législation française.
En cas de litige non résolu à l’amiable entre l’Utilisateur et l’Éditeur, ou l’Adhérent et l’Éditeur, et les Tribunaux de Lyon en France sont compétents pour régler le contentieux.
Privacy Policy
Privacy Policy
Preamble
This Privacy Policy applies to the site: https://www.des-is-it.org
Last updated: June 04, 2022
Respect for your privacy is of utmost importance to D.E.S is it, who is responsible for this site.
The purpose of this Privacy Policy is to provide you with:
- How your personal information is collected and processed. All information that could identify you should be considered personal information. These include your first and last name, your age, your mailing address, your email address, your location or your IP address. "Personal Information" is also used as a synonym for "personal data" as defined in EU Regulation 2016/679;
- What are your rights regarding this information;
- Who is responsible for the processing of personal information collected and processed;
- To whom this information is transmitted;
- The site's policy regarding cookies.
This Privacy Policy supplements the Terms of Use which you can consult on the following page: Terms of Use
ARTICLE 1: Collection of Personal Information
Personal data collected
We collect the following informations:
- Last Name;
- First Name;
- Email address;
- Full mailing address;
- Phone number;
- Copy of identity card;
- Victim type among : DES Mother, DES Son, DES Daughter, DES Grandson, DES Granddaughter, DES Great-grandson, DES Great-granddaughter or a relative;
- Date of birth ;
- Drug brand name, if known ;
- Name and city of the physician who prescribed DES, if known;
- If the member has documentation of DES exposure;
- What heath issues the member has;
- Which of these problems does he/she attribute to DES exposure;
- Additional information;
- If he/she would like or not to be part of our future cohort;
- If he/she accept to be contacted later for a survey;
- Confirmation of the reading and respect of our Bylaws and Rules and Regulations.
How personal data are collected
The personal informations we collect are collected:
- through the membership form;
- through the census form;
- through the newsletter subscription form via the MailChimp emailing platform;
- through the interactivity established between you and our website.
ARTICLE 2 :
Dans le cadre du formulaire de recensement
Les données personnelles collectées par le biais du formulaire de recensement sont utilisées afin de permettre à l'association D.E.S is it de recenser les victimes du diéthylstilbestrol (D.E.S.) résidant en France et dans les départements d'Outre-Mer.
Les données anonymisées nous permettent d'afficher les résultats du recensement en temps réel (graphiques, tableaux, diagrammes...).
Dans le cadre du formulaire de contact
Vous pouvez nous transmettre, si vous le souhaitez, un fichier.
Si vous avez coché la case « Je souhaite recevoir par mail une copie de mon message », vous recevrez une copie du message que vous nous avez adressé.
La collecte de ces données ont pour objectifs : informations, correspondances.
Dans le cadre du formulaire de candidature
Les données personnelles collectées par le biais du formulaire de candidature sont utilisées afin de nous permettre de traiter votre candidature et de vous répondre.
Si vous avez coché la case « Je souhaite recevoir par mail une copie de ma candidature », vous recevrez une copie de la candidature que vous nous avez adressée.
Dans le cadre du formulaire de signalement de problème(s) technique(s)
Les données personnelles collectées par le biais du formulaire de signalement de problème(s) technique(s) sont utilisées afin de nous permettre de résoudre le problème technique signalé et vous informer de sa résolution.
Dans le cadre du formulaire d’adhésion
Vous êtes informé sur notre page d’adhésion qu’un e-mail de la part de D.E.S is it vous sera envoyé à la fin de votre adhésion, vous permettant, si vous le souhaitez, de vous créer un compte adhérent sur notre site.
Dans le cas où vous choisissez de créer un compte adhérent sur notre site, uniquement votre adresse e-mail et votre type de compte sont utilisés pour la création de votre compte adhérent.
En cas de réadhésion, vous recevrez un email de confirmation ; votre accès à l'espace privé reste inchangé, accessible après connexion avec votre identifiant et votre mot de passe.
Dans tous les cas, les données personnelles renseignées lors de votre adhésion sont utilisées à des fins de :
- Enregistrement et mise à jour des données personnelles nécessaires à la gestion administrative du dossier de membre, notamment la gestion des cotisations, et de l’organisation des Assemblées Générales ;
- Réalisation de listes de membres en vue d’afficher sur leur tableau de bord des informations personnalisées ;
- Traitement des sollicitations présentées par les adhérents dans le strict respect de notre objet statutaire.
Dans le cadre du formulaire pour effectuer un don
Le formulaire permettant d’effectuer un don à l’association D.E.S is it, et accessible sur cette page, est fourni par notre partenaire HelloAsso.
Les données relatives aux dons sont gérées par la comptabilité de l’association D.E.S is it.
Dans le cadre du formulaire d’abonnement à la newsletter
Les données personnelles collectées par le biais du formulaire d’abonnement à notre lettre de d’information (newsletter) via la plate-forme d’emailing MailChimp ont pour but :
- Envoi de lettres d’informations
ARTICLE 3 : Proportion et pertinence des données personnelles collectées
Les données personnelles collectées sont strictement nécessaires à l’objectif poursuivi par la collecte. D.E.S is it s’attache à minimiser les données personnelles collectées, à prendre toutes les mesures de précaution nécessaires afin de protéger ces données personnelles, d’en préserver l’intégrité et notamment d’empêcher qu’elles ne soient déformées, endommagées ou communiquées à des tiers non autorisés.
ARTICLE 4 : Partage des données personnelles
Les données personnelles collectées ne peuvent être consultées que par :
- les personnes chargées par les statuts de la gestion de l’association,
- les services de l’association chargés de l’administration et de la gestion des membres,
- les entreprises « sous-traitantes » de l’association au sens des dispositions du RGPD (banque, entreprise en charge de la maintenance informatique, prestataire de service informatique…)
Pour plus d’information, nous vous invitons à consulter :
la politique de confidentialité de notre partenaire HelloAsso: https://www.helloasso.com/confidentialite
la politique de confidentialité de Mailchimp : https://mailchimp.com/legal/privacy/
ARTICLE 5 : Durée de conservation des données personnelles
Les données personnelles collectées sont conservées pendant une durée limitée qui n’excède pas la durée nécessaire aux finalités de collecte.
Dans le cadre du recensement
La durée de conservation de vos données personnelles est de 36 mois (3 ans).
Dans le cas où vous avez accepté d'être contacté ultérieurement, le renouvellement de votre consentement au traitement de vos données pourra vous être redemandé.
Dans le cas contraire, ou si vous refusez de renouveler de votre consentement, vos données pourront être anonymisées afin d'être conservées pour leur valeur statistique.
Dans le cadre de l'adhésion
Nous supprimons de nos systèmes les données personnelles collectées de nos adhérents 30 jours après la fin de la validité de leur adhésion, sauf en cas de renouvellement de l’adhésion qui réactualise les données personnelles collectées et leur durée de conservation.
Si l’adhérent supprime son compte sur le site, il demeure adhérent de l’association D.E.S is it jusqu’à la fin de la validité de son adhésion.
Nous pouvons également conserver les données personnelles collectées au-delà de leur adhésion si nous pensons en toute bonne foi que la loi ou toute autre réglementation exige que nous les conservions et notamment :
Éléments conservés la durée de l'adhésion + 30 jours :
- Les cartes d'adhérents.
Éléments conservés durant la vie de l’association :
- Les statuts et statuts modifiés par ordre chronologique ;
- Liste des dirigeants successifs.
Éléments conservés au minimum 5 ans :
- Convocations de l’assemblée générale ;
- Feuilles d’émargement, pouvoirs ;
- Procès-verbaux d’assemblée ;
- Procès-verbaux du Conseil d’administration.
Éléments conservés au minimum 6 ans :
- Les documents permettant de justifier ce que l’association doit ou non aux services fiscaux, dont les factures et reçus valant paiement de la cotisation.
Éléments conservés au minimum 10 ans :
- Comptes annuels, livres comptables et pièces justificatives.
Éléments conservés 5 ans à compter de la fin de l’adhésion, à des fins statistiques ou de gestion des réclamations :
- La lettre de démission ;
- La lettre d’exclusion ;
- Les courriers liés à la fin de l’adhésion.
Éléments conservés 5 ans, à compter de notre dernière action afin de permettre d’instruire d’éventuelles réclamations :
- Les recours et demandes que nous avons été amenés à traiter pour le compte d’un adhérent durant l’adhésion.
ARTICLE 6 : Sécurité et confidentialité des données personnelles collectées
Des mesures de sécurité physiques, logistiques et organisationnelles appropriées sont prévues pour garantir la confidentialité de vos données personnelles, et notamment éviter tout accès non autorisé.
Les entreprises « sous-traitantes » de l’association sont tenues de respecter la confidentialité de vos informations.
Toutefois, comme aucun mécanisme n'offre une sécurité maximale, une part de risque est toujours présente lorsqu'on utilise Internet pour transmettre des données personnelles.
ARTICLE 7 : Confidentialité et anonymat des réponses aux sondages
Au niveau de votre participation à nos sondages, nous vous rappelons que :
- Votre participation est facultative, vous n’êtes pas obligé-e de répondre.
- Vous ne pouvez participer qu'une fois par sondage.
- Vous ne pouvez participer aux sondages que s'ils s'adressent à votre type de compte.
Au niveau de vos données personnelles (type de compte, participation ou non participation), nous nous engageons :
- À n’utiliser vos données personnelles que dans le but de garantir une méthodologie scientifique de la collecte des données (par exemple dans le but d’empêcher une personne de répondre plusieurs fois ou de garantir un taux de réponse suffisant).
Au niveau de vos réponses à nos sondages nous nous engageons :
- À ce que vos réponses soient anonymisées sitôt votre vote effectuée.
- À ce que vos réponses restent anonymes et qu’il ne soit pas possible d’associer vos réponses à votre identité ou de déduire cette dernière à partir de vos réponses. Même les administrateurs des sondages n’ont accès qu’à des données anonymisées.
- À protéger vos réponses à l’aide des mesures de sécurité physique et informatique adéquates.
- À ce que seuls les participants et les administrateurs puissent accéder aux résultats.
- À ne traiter vos réponses qu’à des fins de recherche.
- À analyser vos réponses de manière scientifique et objective ainsi qu’à donner une image fidèle et représentative de celles-ci
ARTICLE 8 : Responsable du traitement des données personnelles
Le responsable du traitement des données personnelles
Le responsable du traitement des données personnelles est chargé de déterminer les finalités et les moyens mis au service du traitement des données personnelles.
Le responsable du traitement des données personnelles est :
- l’association D.E.S is it
Il peut être contacté de la manière suivante :
- par téléphone au 04 26 65 82 04 du du lundi au vendredi de 10h à 19h30 ou par mail à l'adresse : [email protected]
Obligations du responsable du traitement des données personnelles
Le responsable du traitement des données personnelles s'engage à protéger les données personnelles collectées, à ne pas les transmettre à des tiers sans que vous n'en ayez été informé(e) et à respecter les finalités pour lesquelles ces renseignements ont été collectés.
De plus, le responsable du traitement des données personnelles s'engage à vous aviser en cas de rectification ou de suppression des données personnelles, à moins que cela n'entraîne pour lui des formalités, coûts ou démarches disproportionnés.
Dans le cas où l'intégrité, la confidentialité ou la sécurité de vos données personnelles est compromise, le responsable du traitement s'engage à vous en informer par tout moyen.
ARTICLE 9 : Droit d'opposition et de retrait
Nous nous engageons à vous offrir un droit d'opposition et de retrait quant à vos données personnelles.
Le droit d'opposition s'entend comme étant la possibilité offerte aux adhérents de refuser que leurs données personnelles soient utilisées à certaines fins mentionnées lors de la collecte.
Le droit de retrait s'entend comme étant la possibilité offerte aux adhérents de demander à ce que leurs données personnelles ne figurent plus, par exemple, dans une liste de diffusion.
Afin de formuler une opposition au traitement de vos données personnelles ou demander le retrait de vos données personnelles, vous devez suivre la procédure suivante :
- adresser un courriel au responsable du traitement.
Afin de vous retirer de la liste de diffusion de notre lettre d’information (newsletter), vous pouvez :
- vous désabonner à tout moment en cliquant sur le lien dans le bas de page de nos e-mails envoyés via la plate-forme d’emailing MailChimp.
ARTICLE 10 : Droit d'accès et de rectification
Nous nous engageons à reconnaître un droit d'accès et de rectification aux personnes concernées désireuses de consulter, modifier, voire radier les informations les concernant.
L'exercice de ce droit se fera :
- en adressant un courriel au responsable du traitement.
ARTICLE 11 : Principes généraux en matière de collecte et de traitement des données personnelles en vertu du règlement européen 2016/679
Conformément aux dispositions de l'article 5 du Règlement européen 2106/679, la collecte et le traitement de vos données personnelles respectent les principes suivants :
- Licéité, loyauté et transparence : vos données personnelles ne peuvent être collectées et traitées qu'avec votre consentement. À chaque fois que des données personnelles seront collectées, il sera indiqué que vos données personnelles sont collectées et pour quelles raisons vos données personnelles sont collectées ;
- Finalités limitées : la collecte et le traitement des données personnelles sont exécutés pour répondre à un ou plusieurs objectifs déterminés dans la présente Politique de protection des données personnelles ;
- Minimalisation de la collecte et du traitement des données personnelles : seules les données personnelles nécessaires à la bonne exécution des objectifs poursuivis par le site sont collectées ;
- Conservation des données personnelles réduite dans le temps : les données personnelles sont conservés pour une durée limitée dont vous êtes informés ;
- Intégrité et confidentialité des données personnelles collectés et traités : le responsable du traitement des données personnelles s'engage à garantir l'intégrité et la confidentialité des données personnelles collectés.
Afin d'être licites, et conformément aux exigences de l'article 6 du Règlement européen 2016/679, la collecte et le traitement des données personnelles ne pourront intervenir que s'ils respectent au moins l'une des conditions ci-après énumérées :
- Vous avez expressément consenti au traitement ;
- Le traitement est nécessaire à l'exécution d'un contrat ;
- Le traitement répond à une obligation légale ;
- Le traitement s'explique par la nécessité de sauvegarde des intérêts vitaux de la personne concernée ou d'une autre personne physique ;
- Le traitement peut s'expliquer par une nécessité liée à l'exécution d'une mission d'intérêt public ou qui relève de l'exercice de l'autorité publique ;
- Le traitement et la collecte des données personnelles sont nécessaires aux des intérêts légitimes et privés poursuivis par le responsable du traitement des données personnelles ou par un tiers.
ARTICLE 12 : données personnelles des personnes mineures en vertu du règlement 2016/679
Conformément aux dispositions de l'article 8 du règlement 2016/679, seuls les mineurs âgés de 15 ans ou plus peuvent consentir au traitement de leurs données personnelles.
Si vous êtes un mineur de moins de 15 ans, l'accord d'un représentant légal sera requis afin que des données personnelles puissent être collectées et traitées.
ARTICLE 13 : Fichiers témoins (cookies)
Nous recueillons certaines informations par le biais de fichiers témoins (cookies).
Un témoin est un petit fichier texte qu'un serveur internet peut stocker sur votre machine afin de mémoriser vos préférences de consultation pour un site donné et vous reconnaître lors d'une prochaine visite de ce même site.
Lorsque vous visitez notre site pour la première fois, une fenêtre vous informe de l'utilisation de cookies sur notre site et vous propose de paramétrer vos choix. Ainsi, vous avez la possibilité d'accepter ou de décliner les cookies qui ne sont pas directement nécessaires à l'utilisation de nos services. Un lien vous permettant de modifier vos préférences en matière de cookies est accessible à tout moment en pied de page.
Cookies techniques
Les cookies dits « techniques » sont nécessaires au bon fonctionnement du site et qui ne sont pas soumis au RGPD. Les données y sont encodées et ne sont en aucun cas associées à vos données personnelles.
Mesure d’audience
Ce site utilise Google Analytics. Les informations que nous recueillons par le biais de cookies Google Analytics sont principalement les suivantes :
- Système d'exploitation
- Pages visitées et requêtes
- Heure et jour de connexion
Le recours à de tels fichiers nous permet :
- Amélioration du service
- Statistiques
Protection des formulaires
Afin de sécuriser nos formulaires (contact, candidature...) nous utilisons Google reCaptcha.
Ce service nous permet de vérifier si la personne qui remplit nos formulaires est bien un être humain, et non robot.
Pour plus d’informations sur ce service, veuillez consulter la Politique de confidentialité et les Conditions d’utilisations de Google.
Newsletter
Pour son fonctionnement propre, Mailchimp, présent sur ce site, utilise des fichiers témoins.
Pour en savoir plus, consultez la politique de Mailchimp en matière de cookies : https://mailchimp.com/legal/cookies/
Liste des cookies utilisés sur notre site
| Nom | Fournisseur | À propos | Expiration |
| PHPSESSID | Cookie propre | Faciliter la navigation sur le site en sauvegardant les informations de navigation de page en page uniquement pendant la durée de navigation sur le site. | Session actuelle |
| REMEMBERME | Cookie propre | Ce cookie sert à maintenir la connexion de l'utilisateur ayant coché la case "Se souvenir de moi". | 1 an |
| _ga | Ce nom de cookie est associé à Google Analytics. Ce cookie est utilisé pour distinguer les utilisateurs uniques en attribuant un numéro généré aléatoirement en tant qu'identifiant client. Il est inclus dans chaque demande de page dans un site et utilisé pour calculer les données de visiteurs, de sessions et de campagnes pour les rapports d'analyse de sites. | 2 ans | |
| _gat | Ce nom de cookie est associé à Google Analytics. Il est utilisé pour limiter le débit des demandes, ce qui limite la collecte de données sur les sites à fort trafic. | 10 minutes | |
| _gid | Ce nom de cookie est associé à Google Analytics. Il stocke et met à jour une valeur unique pour chaque page visitée. | Session actuelle | |
|
_GRECAPTCHA |
Détection automatisée d'utilisateurs et amélioration du processus de reconnaissance des formes par les robots. | 6 mois | |
| _abck | Mailchimp | Utilisé pour détecter et défendre contre les attaques par rejeu. Le cookie est nécessaire pour la sécurité et l’intégrité du site. | 1 an |
| bm_sz | Mailchimp | Utilisé avec le BotManager du site. Le BotManager détecte, catégorise et compile des rapports sur les robots potentiels qui essayent d’accéder au site. | 4 heures |
| jwplayerLocalId | ina.fr | De type Local Storage. Utilisé pour déterminer la qualité vidéo optimale en fonction du périphérique du visiteur et des paramètres réseau. | Persistant |
| fr.ina.player.fc | ina.fr | De type Local Storage. Description en attente. | Persistant |
| yt-remote-device-id, yt-player-headers-readable, yt.innertube::nextId, yt-remote-connected-devices, yt.innertube::requests, yt-player-bandaid-host, yt-player-bandwidth | youtube-nocookie.com | De type Local Storage. Description en attente. | Persistant |
|
alreadyShow |
Cookie propre | De type Local Storage. Permet de n'afficher qu'une seule fois la modale vous informant que vous pouvez vous recenser sur le site. | Persistant |
| hasConsent | Cookie propre | Cookie de consentement : il sert à mémoriser les choix relatifs aux cookies des visiteurs sur notre site Web. | 13 mois |
ARTICLE 14 : Conditions de modification de la politique de de protection des données personnelles
La présente Politique de protection des données personnelles peut être consultée à tout moment à l'adresse ci-après indiquée :
Nous nous réservons le droit de la modifier afin de garantir sa conformité avec le droit en vigueur.
Par conséquent, vous êtes invité(e) à venir consulter régulièrement cette politique de confidentialité afin de vous tenir informé(e) des derniers changements qui lui seront apportés.
Toutefois, en cas de modification substantielle de cette politique, vous en serez informé(e) de la manière suivante :
- Par courrier électronique
ARTICLE 15 : Acceptation de la Politique de protection des données personnelles
En navigant sur le site, vous attestez avoir lu et compris la présente Politique de de protection des données personnelles et en acceptez les conditions, en ce qui concerne plus particulièrement la collecte et le traitement de vos données personnelles.
ARTICLE 16 : Législation
Nous nous engageons à respecter les dispositions législatives énoncées dans :
- Loi sur la protection des données personnelles dans le secteur privé, RLRQ c P-39.1 ; et/ou
- Loi sur les protection des données personnelles et les documents électroniques, LC 2000, c 5. ; et
- Règlement général sur la protection des données, Règlement (UE) 2016/679 du Parlement européen et du Conseil du 27 avril 2016 relatif à la protection des personnes physiques à l'égard du traitement des données à caractère personnel et à la libre circulation de ces données, et abrogeant la directive 95/46ÉCE (règlement général sur la protection des données) 2016/279.
Cookie preference settings
This website uses cookies.
Some of our web features use third-party services.
The latter are likely to deposit cookies on your computer to :
- ensure the proper functioning of our website ;
- provide services to visitors of our website ;
- improve our website security ;
- track our website traffic.
While some of them are essential, meaning that they are necessary for the proper functioning of our website, others are "non-essential" cookies, and will only be placed with your consent.
Move the sliders to the right to allow cookies, or to the left to block them.
Please visit our page Privacy Policy for more information about how we use cookies.
You allow :
Essential cookies
These cookies are essential are necessary for the website to function and cannot be disabled.
They are necessary, for example, to save your display setting configuration or your cookie preference settings.
Form security
In order to secure our forms (contact, application...) we use Google reCaptcha.
This service helps us verify that the person filling out our forms is a human being, not a bot.
If you refuse Google reCaptcha cookies, you will not be able to submit the relevant forms to us.
Donation
HelloAsso is a social and solidarity enterprise, that provides its payment systems to our association for free.
Thus, our page Support Us include form provided by HelloAsso.
At the bottom of the form, you will find a link labeled "Gestion des cookies", that allows you to choose which HelloAsso cookies you want to accept.
Certain features of HelloAsso may utilize the services of third-party vendors and business partners.
To learn more, visit the HelloAsso cookie management page.
Video players
INA requires that you accept its cookies to view its videos, in order to offer you targeted ads to your browsing habits.
If you allow INA cookies, cookies will be deposited and you will be able to view the videos. By refusing cookies, you will not be able to access the videos. For more information, please visit the INA "cookies" policy.
On some of our pages, we display Youtube videos Youtube.
In order to block cookies from Youtube, we embed these videos with the "privacy-enhanced" mode. Find out more.
Audience measurement cookies
We use Google Analytics.
These cookies helps us understand the performance of the website.
They provide insights on how the site is currently used and how it can be improved for visitors.
These cookies collect and report on aggregate non-identifiable information. If you disable these cookies, we will not know when you have visited our site.

